Frequently Asked Questions (FAQ) About Batteries
A rechargeable battery, storage battery, secondary cell, or accumulator is a type of electrical battery which can be charged, discharged into a load, and recharged many times, while a non-rechargeable or primary battery is supplied fully charged, and discarded once discharged. It is composed of one or more electrochemical cells. The term “accumulator” is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, nickel cadmium (NiCd), nickel metal hydride (NiMH), lithium ion (Li-ion), and lithium ion polymer (Li-ion polymer).
Rechargeable batteries initially cost more than disposable batteries, but have a much lower total cost of ownership and environmental impact, as they can be recharged inexpensively many times before they need replacing. Some rechargeable battery types are available in the same sizes and voltages as disposable types, and can be used interchangeably with them.
TYPES OF BATTERIES: What are the different types of batteries?
ANSWER:
Batteries are divided in two ways, by application (what they are used for) and construction (how they are built). The major applications are automotive, marine, and deep-cycle. Deep-cycle includes solar electric (PV), backup power, traction, and RV and boat “house” batteries. The major construction types are flooded (wet), gelled, and AGM (Absorbed Glass Mat). AGM batteries are also sometimes called “starved electrolyte” or “dry”, because the fiberglass mat is only 95% saturated with Sulfuric acid and there is no excess liquid.
Flooded may be standard, with removable caps, or the so-called “maintenance free” (that means they are designed to die one week after the warranty runs out). All AGM & gelled are sealed and are “valve regulated”, which means that a tiny valve keeps a slight positive pressure. Nearly all sealed batteries are “valve regulated” (commonly referred to as “VRLA” – Valve Regulated Lead-Acid). Most valve regulated are under some pressure – 1 to 4 psi at sea level. Batteries are typically either Starting, Marine, or Deep-Cycle Batteries
Starting (sometimes called SLI, for starting, lighting, ignition) batteries are commonly used to start and run engines. Engine starters need a very large starting current for a very short time. Starting batteries have a large number of thin plates for maximum surface area. The plates are composed of a Lead “sponge”, similar in appearance to a very fine foam sponge. This gives a very large surface area, but if deep cycled, this sponge will quickly be consumed and fall to the bottom of the cells. Automotive batteries will generally fail after 30-150 deep cycles if deep cycled, while they may last for thousands of cycles in normal starting use (2-5% discharge).Marinebatteries are usually a “hybrid”, and fall between the starting and deep-cycle batteries, though a few (Rolls-Surrette and Concorde, for example) are true deep cycle. In the hybrid, the plates may be composed of Lead sponge, but it is coarser and heavier than that used in starting batteries. It is often hard to tell what you are getting in a “marine” battery, but most are a hybrid. Starting batteries are usually rated at “CCA”, or cold cranking amps, or “MCA”, Marine cranking amps – the same as “CA”. Any battery with the capacity shown in CA or MCA may or may not be a true deep-cycle battery. It is sometimes hard to tell, as the term deep cycle is often overused – we have even seen the term “deep cycle” used in automotive starting battery advertising. CA and MCA ratings are at 32 degrees F, while CCA is at zero degree F. Unfortunately, the only positive way to tell with some batteries is to buy one and cut it open – not much of an option.
It will not hurt a deep cycle battery to be used as a starting battery, but for the same size battery they cannot supply as much cranking amps as a regular starting battery and is usually much more expensive.
BATTERY LIFESPAN: How long do batteries last on average?
ANSWER:
The lifespan of a deep cycle battery will vary considerably with how it is used, how it is maintained and charged, temperature, and other factors. In extreme cases, it can vary to extremes – we have seen L-16’s killed in less than a year by severe overcharging and water loss, and we have a large set of surplus telephone batteries that sees only occasional (10-15 times per year) heavy service that were just replace after 35+ years. We have seen gelled cells destroyed in one day when overcharged with a large automotive charger. We have seen golf cart batteries destroyed without ever being used in less than a year because they were left sitting in a hot garage or warehouse without being charged. Even the so-called “dry charged” (where you add acid when you need them) have a shelf life of 18 months at most. (They are not totally dry – they are actually filled with acid, the plates formed and charged, then the acid is dumped out).
These are some typical (minimum – maximum) typical expectations for batteries if used in deep cycle service. There are so many variables, such as depth of discharge, maintenance, temperature, how often and how deep cycled, etc. that it is almost impossible to give a fixed number.
- Starting: 3-12 months
- Marine: 1-6 years
- Golf cart: 2-7 years
- AGM deep cycle: 4-8 years
- Gelled deep cycle: 2-5 years
- Deep cycle (L-16 type etc): 4-8 years
- Rolls-Surrette premium deep cycle: 7-15 years
- Industrial deep cycle (Crown and Rolls 4KS series): 10-20+ years.
- Telephone (float): 2-20 years. These are usually special purpose “float service”, but often appear on the surplus market as “deep cycle”. They can vary considerably, depending on age, usage, care, and type.
- NiFe (alkaline): 5-35 years
- NiCad: 1-20 years
BATTERY RATINGS: How are batteries rated and what do the ratings mean in battery selection?
ANSWER:
The most common battery rating is the AMP-HOUR RATING. This is a unit of measurement for battery capacity, obtained by multiplying a current flow in amperes by the time in hours of discharge. (Example: A battery which delivers 5 amperes for 20 hours delivers 5 amperes times 20 hours, or 100 ampere-hours.)
Manufacturers use different discharge periods to yield an different Amp-Hr. Rating for the same capacity batteries, therefore, the Amp-Hr. Rating has little significance unless qualified by the number of hours the battery is discharged. For this reason Amp-Hour Ratings are only a general method of evaluating a battery’s capacity for selection purposes. The quality of internal components and technical construction within the battery will generate different desired characteristics without effecting its Amp-Hour Rating. For instance, there are 150 Amp-Hour batteries that will not support an electrical load overnight and if called upon to do so repetitively, will fail early in their life. Conversely, there are 150 Amp-Hour batteries that will operate an electrical load for several days before needing recharging and will do so for years. The following ratings must be examined in order to evaluate and select the proper battery for a specific application: COLD CRANKING AMPERAGE and RESERVE CAPACITY are ratings used by the industry to simplify battery selection.
Batteries come in all different sizes. Many have “group” sizes, which is based upon the physical size and terminal placement. It is NOT a measure of battery capacity. Typical BCI codes are group U1, 24, 27, and 31. Industrial batteries are usually designated by a part number such as “FS” for floor sweeper, or “GC” for golf cart. Many batteries follow no particular code, and are just manufacturers part numbers. Other standard size codes are 4D & 8D, large industrial batteries, commonly used in solar electric systems.
Some common battery size codes used are: (ratings are approximate)
| U1 | 34 to 40 Amp hours | 12 volts |
| Group 24 | 70-85 Amp hours | 12 volts |
| Group 27 | 85-105 Amp hours | 12 volts |
| Group 31 | 95-125 Amp hours | 12 volts |
| 4-D | 180-215 Amp hours | 12 volts |
| 8-D | 225-255 Amp hours | 12 volts |
| Golf Cart & T-105 | 180 to 225 Amp hours | 6 volts |
| L-16, L16HC etc. | 340 to 415 Amp hours | 6 volts |
Gelled Electrolyte
Gelled batteries, or “Gel Cells” contain acid that has been “gelled” by the addition of Silica Gel, turning the acid into a solid mass that looks like gooey Jell-O. The advantage of these batteries is that it is impossible to spill acid even if they are broken. However, there are several disadvantages. One is that they must be charged at a slower rate (C/20) to prevent excess gas from damaging the cells. They cannot be fast charged on a conventional automotive charger or they may be permanently damaged. This is not usually a problem with solar electric systems, but if an auxiliary generator or inverter bulk charger is used, current must be limited to the manufacturers specifications. Most better inverters commonly used in solar electric systems can be set to limit charging current to the batteries.
Some other disadvantages of gel cells is that they must be charged at a lower voltage (2/10th’s less) than flooded or AGM batteries. If overcharged, voids can develop in the gel which will never heal, causing a loss in battery capacity. In hot climates, water loss can be enough over 2-4 years to cause premature battery death. It is for this and other reasons that we no longer sell any of the gelled cells except for replacement use. The newer AGM (absorbed glass mat) batteries have all the advantages (and then some) of gelled, with none of the disadvantages
AGM (Absorbed Glass Mat) Batteries
A newer type of sealed battery uses “Absorbed Glass Mats”, or AGM between the plates. This is a very fine fiber Boron-Silicate glass mat. These type of batteries have all the advantages of gelled, but can take much more abuse. We sell the Concorde (and Lifeline, made by Concorde) AGM batteries. These are also called “starved electrolyte”, as the mat is about 95% saturated rather than fully soaked. That also means that they will not leak acid even if broken. AGM batteries have several advantages over both gelled and flooded, at about the same cost as gelled:
Since all the electrolyte (acid) is contained in the glass mats, they cannot spill, even if broken. This also means that since they are non-hazardous, the shipping costs are lower. In addition, since there is no liquid to freeze and expand, they are practically immune from freezing damage.
Nearly all AGM batteries are “recombinant” – what that means is that the Oxygen and Hydrogen recombine INSIDE the battery. These use gas phase transfer of oxygen to the negative plates to recombine them back into water while charging and prevent the loss of water through electrolysis. The recombining is typically 99+% efficient, so almost no water is lost.
The charging voltages are the same as for any standard battery – no need for any special adjustments or problems with incompatible chargers or charge controls. And, since the internal resistance is extremely low, there is almost no heating of the battery even under heavy charge and discharge currents. The Concorde (and most AGM) batteries have no charge or discharge current limits.
AGM’s have a very low self-discharge – from 1% to 3% per month is usual. This means that they can sit in storage for much longer periods without charging than standard batteries. The Concorde batteries can be almost fully recharged (95% or better) even after 30 days of being totally discharged.
AGM’s do not have any liquid to spill, and even under severe overcharge conditions hydrogen emission is far below the 4% max specified for aircraft and enclosed spaces. The plates in AGM’s are tightly packed and rigidly mounted, and will withstand shock and vibration better than any standard battery.
Even with all the advantages listed above, there is still a place for the standard flooded deep cycle battery. AGM’s will cost about 1.5 to 2 times as much as flooded batteries of the same capacity. In many installations, where the batteries are set in an area where you don’t have to worry about fumes or leakage, a standard or industrial deep cycle is a better economic choice. AGM batteries main advantages are no maintenance, completely sealed against fumes, Hydrogen, or leakage, non-spilling even if they are broken, and can survive most freezes. Not everyone needs these features.
COLD CRANKING AMPERAGE: How does the Cold Cranking Amperage rating help me select a battery?
ANSWER:
(CCA) is the maximum amperes that can be continuously removed from a battery for 30 seconds at 0°F before its voltage drops to unusable levels. A 550 CCA battery can supply 550 amperes for 30 seconds at 0°F. This rating is only useful in the selection of engine starting batteries.
NOTE: Do not confuse Cold Cranking Amperage (CCA) with Marine Cranking Amperage (MCA) or Cranking Amperage (CA). MCA and CA is a higher battery rating measured at warmer temperatures.
RESERVE CAPACITY: What does the Reserve Capacity rating mean and how does it apply to deep cycle batteries?
ANSWER:
Reserve capacity is the number of minutes a battery can maintain a useful voltage under a 25 ampere discharge. The higher the minute rating, the greater the battery’s ability to run lights, pumps, inverters, and electronics for a longer period before recharging is necessary. The 25 Amp. Reserve Capacity Rating is more realistic than Amp-Hour or CCA as a measurement of capacity for deep cycle service. Batteries promoted on their high Cold Cranking Ratings are easy and inexpensive to build. The market is flooded with them, however their Reserve Capacity, Cycle Life (the number of discharges and charges the battery can deliver) and Service life are poor. Reserve Capacity is difficult and costly to engineer into a battery and requires higher quality cell materials.
For instance, Rolls, Surrette and Lifeline use thicker lead grids (the plate’s skeletal structure) to support additional positive plate oxides which are compressed into a denser form in order to add battery reactive material for greater Reserve Capacity and Cycling Performance. In addition, these plates are separated by indestructible separators. These mats hold the active oxides tightly in place during the cubical plate expansion which occurs during deep discharging, instead of allowing the oxides to shed off and precipitate to the bottom of the battery. Construction materials such as those raise the Reserve Capacity of a battery and increase the battery’s Cycle Life.
CYCLE LIFE: What is battery cycle life?
ANSWER:
One cycle of a battery is a discharge from full charge to full discharge and a return to full charge again. The total number of cycles a battery can perform before failure is called its Cycle Life. Most battery manufacturers will not discus the Cycle Life of their product. Many advertised Deep Cycle batteries have not been tested, or, which is the case with cranking batteries, were never designed for long Cycle Life .
DEEP CYCLE BATTERIES: What is the difference between deep cycle batteries and starting batteries?
ANSWER:
Unfortunately, the term Deep Cycle has been overused by the battery industry as a sales tool to imply a heavy duty product. This has led to confusion and difficulty in battery selection. One must understand that any battery may be termed deep cycle as all batteries may be fully discharged and charged. However, a true deep cycle battery, such as Rolls or Lifeline, is capable of thousands of these hard cycles during its life without losing its capacity. Comparatively, many advertised deep cycle batteries composed of thin plates, excessively porous separators, and low density plate oxides will suffer permanent capacity loss after a few dozen cycles and will shortly sulfate or shed plate material and fail. Batteries without substantial materials designed for true deep-cycling will lose more than half of their capacity after only a few cycles. A 200 Amp-hour battery will shortly become a 100 Amp-hour battery for the remainder of its shortened service life. What initially may seem to be an inexpensive battery to purchase, now costs twice as much per Amp-hour. True Deep cycle batteries will perform well as cranking batteries, however, cranking batteries will not survive deep cycle use.
Deep cycle batteries can be used in any application and exhibit a long service life, while cranking batteries are limited to starting applications only. Cranking batteries exhibit poor service life in cycling applications.
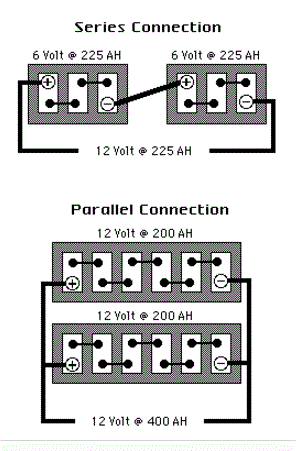
INCREASING CAPACITY THROUGH SERIES AND PARALLEL CONNECTIONS: What is the difference between series battery connections and parallel battery connections and how do they increase battery capacity and voltage?
ANSWER:
In the SERIES CONNECTION, batteries of like voltage and Amp-Hour capacity are connected to increase the Voltage of the bank. The positive terminal of the first battery is connected to the negative terminal of the second battery and so on, until the desired voltage is reached. The final Voltage is the sum of all battery voltages added together while the final Amp-Hours remains unchanged. The bank’s Voltage increases while its Amp-Hours, Cranking Performance and Reserve Capacity remain unchanged.
In the PARALLEL CONNECTION, batteries of like voltages and capacities are connected to increase the capacity of the bank. The positive terminals of all batteries are connected together, or to a common conductor, and all negative terminals are connected in the same manner. The final voltage remains unchanged while the capacity of the bank is the sum of the capacities of the individual batteries of this connection. Amp-Hours Cranking Performance and Reserve Capacity increases while Voltage does not.
BATTERY MAINTENANCE: Does overcharging damage batteries?
ANSWER:
OVERCHARGING is the most destructive element in battery service. Usually the boater is not aware that this is occurring as he believes his alternator or battery charger is “automatic.” Unfortunately, these automatic circuits are sensitive to voltage surges, heat, direct lightening strikes and indirect lightening electromagnetic influences and could fail or shift their calibration. When they fail, overcharging begins to effect the batteries. During overcharging, excessive current causes the oxides on the plates of the battery to “shed” and precipitate to the bottom of the cell and also heat the battery, thus removing water from the electrolyte. Once removed, this material (which represents capacity) is no longer active in the battery. In addition, the loss of water from the electrolyte may expose portions of the plates and cause the exposed areas to oxidize and become inactive, thus reducing additional capacity. Sealed batteries are not immune from the same internal results when overcharged. In fact, sealed recombination absorption and gel batteries are particularly sensitive to overcharging. Once moisture is removed from the battery, it cannot be replaced. Portions of the battery damaged due to overcharging are irretrievable. However, if detected early, corrective adjustments to the charging device will save the undamaged portion of the battery. Initial signs of overcharging are excessive usage of water in the battery, continuously warm batteries, or higher than normal battery voltages while under the influence of the charger. If overcharging is suspected, correct immediately.
OVER-DISCHARGING: Does over-discharging damage batteries?
ANSWER:
OVER-DISCHARGING is a problem which originates from insufficient battery capacity causing the batteries to be overworked. Discharges deeper than 50% (in reality well below 12.0 Volts or 1.200 Specific Gravity) significantly shorten the Cycle Life of a battery without increasing the usable depth of cycle. Infrequent or inadequate complete recharging can also cause over-discharging symptoms called SULFATION. Despite that charging equipment is regulating back properly, over-discharging symptoms are displayed as loss of battery capacity and lower than normal specific gravity. Sulfation occurs when sulfur from the electrolyte combines with the lead on the plates and forms lead-sulfate. Once this condition becomes chronic, marine battery chargers will not remove the hardened sulfate. Sulfation can usually be removed by a proper desulfation or equalization charge with external manual battery chargers. To accomplish this task, the flooded plate batteries must be charged at 6 to 10 amps. at 2.4 to 2.5 volts per cell until all cells are gassing freely and their specific gravity returns to their full charge concentration. Sealed AGM batteries should be brought to 2.35 volts per cell and then discharged to 1.75 volts per cell and their this process must be repeated until the capacity returns to the battery. Gel batteries may not recover. In most cases, the battery may be returned to complete its service life.
CHARGING Alternators and float battery chargers including regulated photo voltaic chargers have automatic controls which taper the charge rate as the batteries come up in charge. It should be noted that a decrease to a few amperes while charging does not mean that the batteries have been fully charged. Battery chargers are of three types. There is the manual type, the trickle type, and the automatic switcher type.
BATTERY EVALUATION: How can I evaluate the health and charge state of a battery?
ANSWER:
Routine battery examinations divulge irregularities in the charging system as well as in the batteries. The principle method is to examine the electrochemistry of the battery through hydrometric electrolyte inspection. As previously discussed, this important examination cannot be accomplished with sealed absorption or gel batteries. Voltage readings alone require experience to interpret. Hydrometric readings will uncover early warnings of overcharging or over-discharging before batteries are damaged. The state-of-charge and reliability of a lead acid battery can best be determined by the specific gravity of the electrolyte measured directly with a common bulb-type hydrometer with a glass float. We do not recommend the ball float type hydrometer. Specific gravity is a unit of measurement for determining the sulfuric acid content of the electrolyte. The recommended fully charged specific gravity of marine batteries is 1.255 to 1.265 taken at 80°F. More than .025 spread in readings between fully charged cells indicates that the battery may need an equalization charge. If this condition persists, the cell is failing and the battery should be replaced. Since water has a value of 1.000, electrolyte with a specific gravity of 1.260 means it is 1.260 times heavier than pure water while pure concentrated sulfuric acid has a specific gravity of 1.835.
The following table illustrates typical specific gravity values for a cell in various stages of charge:
100% Charged.......1.255 - 1.260 Sp. Gr. 75% Charged.......1.220 - 1.225 Sp. Gr. 50% Charged.......1.185 - 1.190 Sp. Gr. 25% Charged.......1.150 - 1.155 Sp. Gr. 0% Charged.......1.115 - 1.120 Sp. Gr.
Temperature compensation of hydrometric readings is usually unnecessary unless the battery is extremely hot or cold, however, after hard charging or discharging, you may want to add or subtract points of Specific Gravity based on the table.
